malaria vaccine trials
Ongoing Research Malaria Rota Research. Meanwhile BioNTech has unveiled plans to build a modern facility in Rwanda to utilize mRNA technology to develop new preventives against malaria tuberculosis and other diseases.
Vaccines Free Full Text Progress In The Development Of Subunit Vaccines Against Malaria Html
The presence of the parasite in the blood to confirm that you have malaria.

. FluCamp clinical trials offer up to 3750 in compensation apply online today. The most effective malaria vaccine is R21Matrix-M with a 77 efficacy rate shown in initial trials and significantly higher antibody levels than with the. It requires four injections.
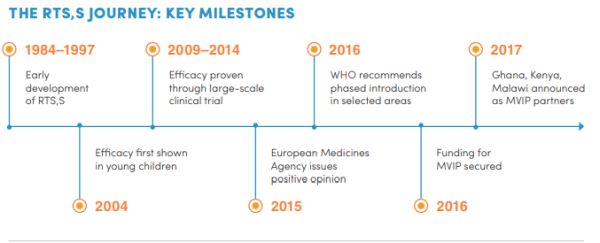
A vaccine against malaria called RTSSAS01 RTSS was approved by European regulators in 2015. Blood tests can indicate. The Malaria Vaccine Implementation Programme is coordinated by WHO and supported by in-country and international partners including PATH UNICEF and GSK which is donating up to 10 million doses of the vaccine for the pilot.
The Covid-19 vaccine trials have not yet been completed and therefore the results cannot be peer-reviewed. On Thursday it administered the first doses of a shot it co-developed with the International. Preclinical safety and efficacy studies with ALF LFA ALFQ and ALFQA are discussed in preparation for upcoming vaccine trials targeting malaria HIV-1 bacterial diarrhea and opioid addiction.
While effective tools have been and will continue to be developed to combat malaria inevitably over time the parasites and mosquitoes will evolve means to circumvent those tools if used in isolation or used ineffectively. Which type of malaria parasite is causing your symptoms. A new HIV vaccine is in phase one trials.
This parasite affects at least 70 million people and is widespread in Africa and Brazil. Furthermore vaccine trials involving younger children and testing different vaccines are underway in various countries1 While most of these trials are currently conducted in high-income countries it is likelyand necessarythat COVID-19 vaccine trials involving children will increasingly be extended to lowermiddle-income countries. We are working on a monovalent vaccine formulated on alum and a second adjuvant.
Research continues with other malaria vaccines. Also a genetically engineered vaccine that prevents infection for the intestinal form of the disease caused by Schistosoma mansoni. With support from the Bill Melinda Gates Foundation research is being conducted to leverage CureVacs prophylactic vaccine technology to develop mRNA-based vaccines to prevent infectious diseases that disproportionately affect people in the worlds poorest countries.
Sm-TSP-2 Schistosomiasis Vaccine. Malaria is a difficult disease to control largely due to the highly adaptable nature of the vector and parasites involved. An efficacious malaria vaccine would be an important tool to combat the enormous socioeconomic burden caused by malaria.
To diagnose malaria your doctor will likely review your medical history and recent travel conduct a physical exam and order blood tests. A malaria vaccine is a vaccine that is used to prevent malariaThe only approved vaccine as of 2021 is RTSS known by the brand name Mosquirix. Novavaxs promise doesnt stop at the COVID-19 vaccine.
Regulatory expectations adopted by the ECBS in October 2016. On October 6 th the World Health Organization WHO formally recommended the RTSS malaria vaccine for broader use external icon among children in sub-Saharan Africa and in other regions with moderate to high malaria transmission. And Moderna says it will fund an mRNA vaccine facility in Africa possibly at sites in Senegal Rwanda or South Africa.
Help us drive flu asthma covid-19 RSV vaccine development through clinical trial research discovery. It follows a review of two years of piloting studies of the vaccine in three sub-Saharan African countries with a high. The document on human challenge trials should be read in conjunction with the updated Guidelines on clinical evaluation of vaccines.
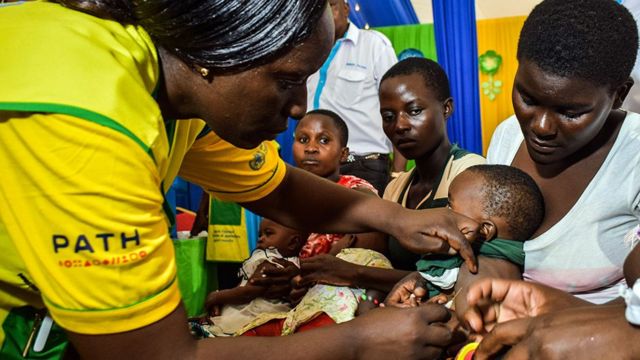
Trials need to be well defined by the NRAs and vaccine developers and manufacturers need to be aware of regulatory expectations. Bed nets the most widespread preventive measure cut malaria deaths in children under 5. As of 2019 it is undergoing pilot trials in 3 sub-Saharan African countries Ghana Kenya and Malawi as part of the WHOs Malaria Vaccine Implementation Programme MVIP.
Waning vaccine efficacy against a clinical endpoint has been documented in randomized controlled trials of several vaccines including the RTSSAS01 malaria vaccine and 2 similar killed whole-cell oral cholera vaccines. It is the first malaria vaccine to be recommended by the global health body. Malaria research is littered with vaccine candidates that never made it past clinical trials.
A collaborative CDC and KEMRI trial in western Kenya has shown that the PfSPZ Vaccine is safe and well tolerated in infants and young children. Moderna has begun early-stage clinical trials of an HIV mRNA vaccine the company announced this week. The introduction of ALF in the 1980s stimulated commercial interest in vaccines to infectious diseases and therapeutic vaccines to.
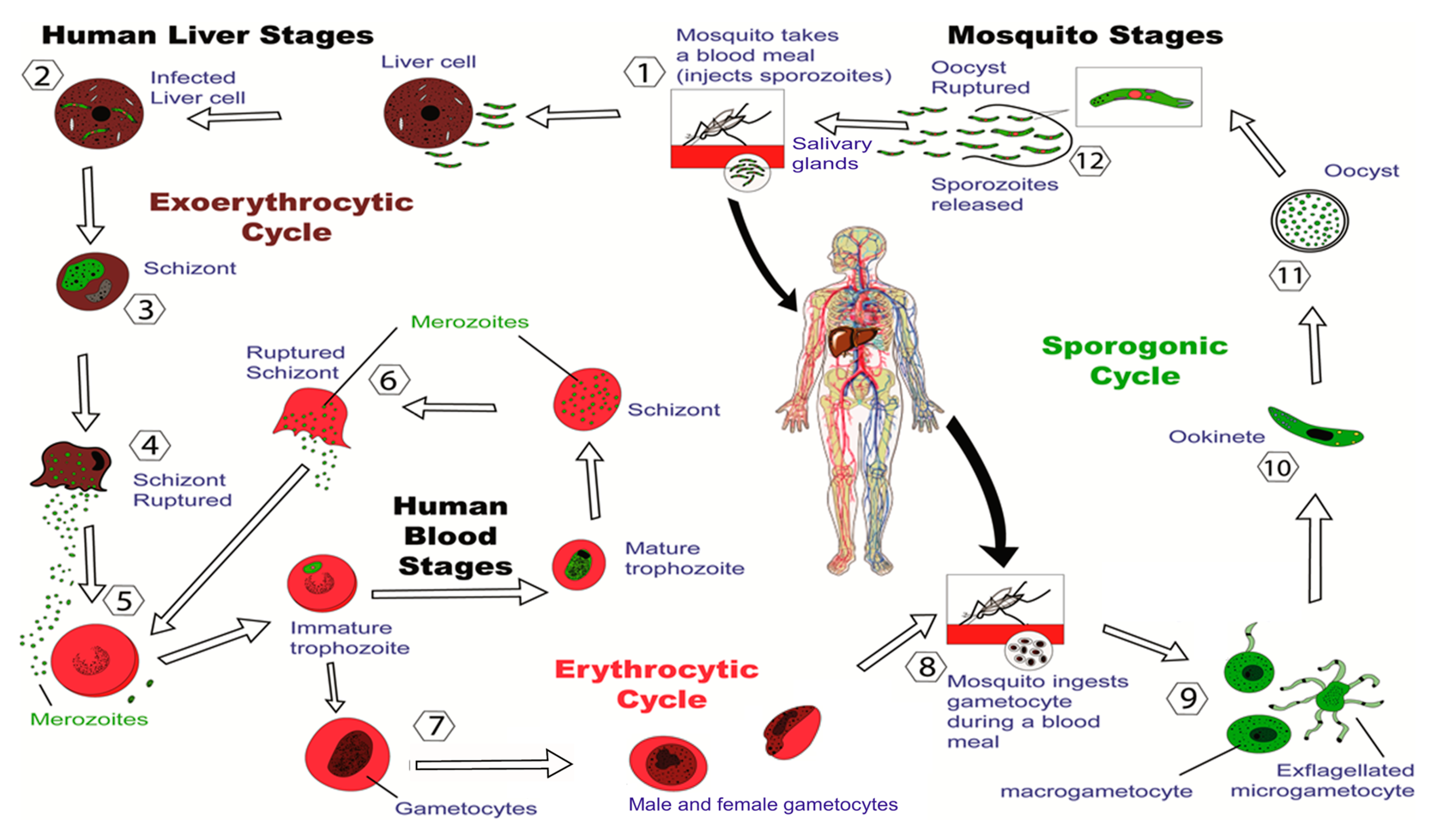
Last week the company reported positive results from a phase 2 trial for its malaria vaccine candidate R21 in a. Malaria can be caused by several species of Plasmodium parasites each of which has a complex life cycle see illustrationResearch in recent decades has shed light on many aspects of Plasmodium biology broadening understanding of how parasites interact with the human immune system cause human disease and are transmitted by mosquitoesStill in these fundamental. The clinical trials of a coronavirus vaccine candidate being developed in Australia have been scrapped after trial participants returned.
RTSSAS01 is the first malaria vaccine to be tested in Phase 3 clinical trials and the first to be assessed in routine immunization programs in malaria-endemic areas. To reduce malaria infections world health programs distribute preventive drugs and insecticide-treated bed nets to protect people from mosquito bites. The RTSS malaria vaccine is the result of 30 years of research and development by GSK and through a partnership with.
But scientists arent holding their breath just yet Years of false starts have made hopeful researchers wary of. This is the first time ever that a vaccine has been recommended to combat malaria a disease that has killed billions of people globally over. Volunteer for paid flu asthma clinical trials in London UK.
The data collection and analysis are ongoing in order to allow up to two years of follow up on participants. The Covid-19 vaccine trials have been published in peer reviewed journals. The World Health Organization has recommended a malaria vaccine for use in children who live in countries with high numbers of malaria cases.
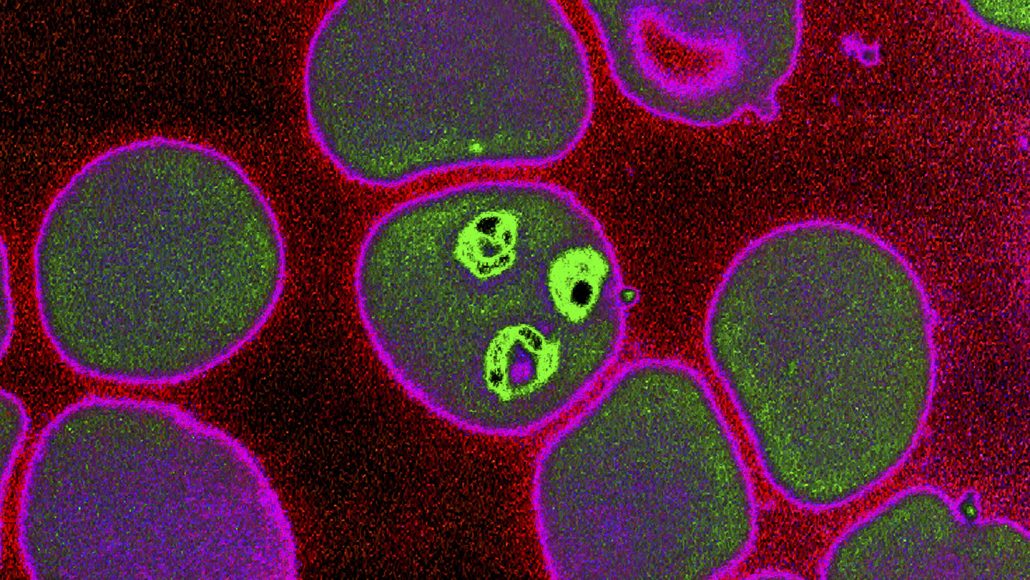
Recent trials have shown that the irradiated whole sporozoite PfSPZ Vaccine made by Sanaria is safe and well tolerated and had promising protection against malaria when administered intravenously.

Who Recommends Broad Roll Out Of Gsk S Malaria Vaccine For Children

The History Of The Rts S As01 Malaria Vaccine Trial The Lancet

Malaria Vaccines Malaria Site

World S First Malaria Vaccine Launched In A Pilot Program

Cryoport Developing Cold Chain Logistic Models For Malaria Vaccine

Oxford Malaria Vaccine Proves Highly Effective In Burkina Faso Trial Malaria The Guardian

R21 Matrix M Malaria Vaccine Becomes First To Reach 75 Efficacy Target
A Malaria Vaccine With Live Parasites Shows Promise In A Small Trial Science News

Malaria Vaccine Candidates In Clinical Development Data Sources For Download Scientific Diagram
World S First Malaria Vaccine Pilot Program Launched In Malawi Food Drugs Healthcare Life Sciences Malawi
Malaria Vaccine World Health Organization Approve Malaria Vaccine For Africa Bbc News Pidgin
Schedule On The Malaria Vaccine Development For Approximately 50 Years Download Scientific Diagram

Seven Year Efficacy Of Rts S As01 Malaria Vaccine Among Young African Children Nejm
/news/malaria-vaccine-edctp-news.jpg?sfvrsn=6ba0bfae_5)
Scientists Share Data From First Who Recommended Malaria Vaccine

Efficacy Of A Low Dose Candidate Malaria Vaccine R21 In Adjuvant Matrix M With Seasonal Administration To Children In Burkina Faso A Randomised Controlled Trial The Lancet